|
Three quite different wet end chemical control methods are commonly used to treat paper machine contaminants: stabilization, detackification, and fixation/removal. However, these methods are not commonly used together, since they may conflict with one another. A new approach combining these methods has been developed, and results based on commercial trials have shown that it is considerably more effective on commercial paper machines than conventional methods.
Paper machine contaminants come in many forms, and the first step in effectively managing them is to optimize mechanical screening and cleaning systems to remove them before they are a problem. This also minimizes contaminant particle size, making them more easily passed through the system or addressed by chemical means. However, problematic contaminants, such as stickies from secondary fiber and coated broke and natural wood pitch and resins, are not completely removed. These materials can cause paper quality defects, as well as machine deposits and foul machine clothing. If not managed well, they can lead to poor runnability and less production.
Traditionally, once passed through screening and cleaning, these materials are managed chemically. In order to understand how these methods perform, a review of the parameters affecting deposition and fouling is helpful.
|
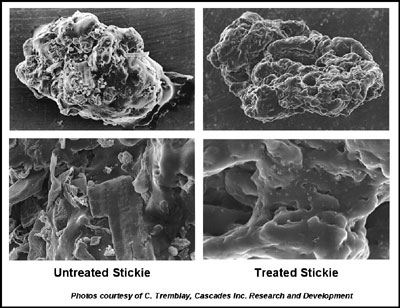
Lab tests demonstrated that treated stickies have a clean, smooth surface, indicating the addition of structured protein detackified the surface.
|
PARAMETERS AFFECTING DEPOSITION. Numerous factors affect the extent to which contaminant deposition and fouling occur. They include temperature, pH, system closure, fiber source, and the nature of the surface the deposit forms on. These factors can be encompassed by four parameters: (1) content or amount of contaminant in the system; (2) depositability; (3) colloidal stability; and (4) surface affinity for deposition.
Content. If the amount of contaminant is reduced, it is obvious there will be less available for deposition. The converse is true as well. Managing the fiber source to limit contaminants coming into the system, as well as screening and cleaning, are commonly used to manage content. Colloidal contaminants may be increased and concentrated by recycling and reusing white water for system closure. Purging contaminants from wastewater streams or through clarification can reduce content. Additionally, retaining and binding colloidal materials during sheet formation and then removing them with the sheet reduces content.
Table 1. Addition of the structured protein led to significantly lower solvent use and fewer washups.
|
|
Trial Condition
|
% Reduction in Solvent Use
|
Average Solvent Washes Per Day
|
|
Pre-trial
|
----
|
6.6
|
|
Initial addition point
|
64
|
2.4
|
|
Subsequent addition point
|
94
|
0.4
|
|
Depositability. Depositability may be thought of as the tackiness or adhesive property of the contaminants, which is influenced by chemical composition, viscosity, temperature, and its surface. For example, wood is aged to increase the oxidation of extractives and decrease the depositability of the pitch. Hot melt adhesives and latex binders flow more easily and gain tack as temperature is increased. Therefore, wet end operating and dryer temperature control may reduce deposition. Applying a chemical barrier (coating) to the contaminant will also lower tackiness and reduce depositability.
Colloidal stability. Maximizing contaminant colloidal stability allows it to pass through the process without agglomerating or depositing. Stability is maintained by limiting mechanical shear, thermal agitation, repulsive electrostatic forces, and repulsive surface properties of suspended particles. Changes in concentration due to introduction of fresh water or destabilizing cations may lead to deposition. Temperature shock ought to also be guarded against, as this will influence stability as well.
Factors contributing to stability include small particle size, dissolved organic materials released during pulping, and stability-enhancing surfactants or dispersants. Smaller particles are more easily stabilized than larger particles. High-temperature mechanical dispersion and solvent chemical treatments, for example, can minimize particle size and maximize stability.
Surface affinity. The more receptive a paper machine surface is to the contaminant, the more likely deposits will form. Special Uhle box covers and Teflon coatings for dryer cans can limit deposits. Forming fabrics, felts, and rolls can be sprayed with cationic polymers that form a coating to reduce deposition.
COMMON CHEMICAL APPROACHES. Paper mills commonly use three chemical methods to manage contaminants such as pitch and stickies. These methods are stabilization, detackification, and fixation and removal.
Stabilization. Surfactants and dispersants may be applied to chemically enhance colloidal stability. Surfactants are generally nonionic or anionic, and the dispersants are strongly anionic. Unfortunately, this approach may reduce the effectiveness of an additive used to retain the colloidal material in the sheet.
Detackification. In detackification, a chemical is used to build a boundary layer of water around the contaminant. Mineral absorbents may also function by detackification, creating a physical boundary around the deposit. Both rely on surface attraction to a hydrophobic contaminant and function to decrease depositability. Stability may be enhanced with chemical treatment, but particle size will be increased by mineral treatments, which, in theory, would decrease the colloidal stability of the contaminants.
Fixation and removal. Cationic polymers are used to combine with and retain, or "fix," contaminants onto the fiber, thus removing them from the system. This approach relies on anionic contaminants reacting with the cationic polymer. This method also often removes anionic trash, which can lead to improved chemical additive efficiency. However, if the characteristics of the polymer are not matched to the papermaking system, deposition may be aggravated rather than reduced.
Fixation combines the cationic polymer with the contaminant, which is fixed to the fiber, thus destroying colloidal stability. If the complexes are not fixed, they will concentrate in the system, which can lead to deposition. Alum, starches, and some low molecular weight coagulants can neutralize anionic trash and detrimental substances or form complexes. However, they may not carry sufficient cationic charge and/or molecular weight to fix to the fiber. Further, the contaminant particle size must be small enough to strongly affix stickies to the fibers or fines, or they can be pulled from the sheet or redeposited.
COMBINING CHEMICAL METHODS. A new approach has been developed that combines an amphoteric, surface-active, structured protein with a highly-charged cationic polymer. The structured protein is able to both increase contaminant stability and reduce tackiness. The real key, however, is the effect achieved when the structured protein is used together with a cationic polymer to retain the contaminants with the web.
The structured protein performs its function through adsorption onto colloidal material, thereby enhancing colloidal stability and modifying the contaminant surface to reduce adhesion.
The protein's adsorption onto the surface of hydrophobic materials can be measured as a reduction of the zeta potential of a colloidal suspension. Test results show the reduction in zeta potential of colloidally dispersed polystyrene as addition of the structured protein is increased. This indicates the adhesion of the structured protein onto the hydrophobic polystyrene and its attraction to hydrophobic surfaces.
Preferably, this adsorption is strong enough so as to not to be easily washed off or displaced from the surface. This would also prevent the structured protein from being removed by the effects of dilution, refiners, or pumps. Test data comparing a commercial detackifier to the structured protein indicate the protein has a greater tenacity to adhere to the hydrophobic surface.
Colloidal stability is a function of the dispersive forces between contaminant particles. Since charge is reduced by adsorption of the protein, the stability between contaminant particles is more a function of interfacial or steric stabilization. Test data illustrate that if contaminants are treated with the structured protein, greater colloidal stability can be expected. This reduces the tendency of treated contaminants to agglomerate and deposit.
A key to the functionality of the structured protein is surface modification to reduce tack and depositability. Its adsorption onto the surface allows for the building of physical and water layers around the contaminant. These layers interfere with the adhesion of the contaminants to other surfaces. This can be demonstrated by measuring the force required to separate adhesive surfaces with and without the presence of a "detackifier." The percentage reduction in the required tack, or force, is termed the percent detackification. The relative effectiveness of detackification versus a commercial detackifier was studied. Data confirmed that addition of small amounts of structured protein almost eliminated the tack of the adhesives.
In order to reduce the content of contaminants in a papermaking system, the complex formed by the structured protein and contaminant must be able to be retained. The retention of the complex in the sheet purges contaminants, lowering the concentration or content of the contaminants in the system.
Reaction with cationic polymers to fix or bridge a complex onto a fiber surface is a common method of retention. Fixation by cationic polymers has been demonstrated in both the laboratory and in a mill production environment. As an example, tests were conducted in a mill producing bag paper from 100% recycled double lined kraft and clippings. The results demonstrated the effect that the combination of structured protein and fixative had on colloidal material. A percent reduction in the turbidity of the filtrate is used to correlate with colloidal contaminant content and as a measure of colloidal retention. An accelerating reduction in turbidity, above that of cationic polymer alone, was seen in the presence of a constant concentration of structured protein and increasing cationic fixative addition.
Mill trials verified the usefulness of this new approach. It has been successfully applied and is currently in use in recycled corrugating medium and tissue grade mills. The following cases demonstrate effective deposit control and reductions in colloidal loadings.
CASE HISTORY: CORRUGATING MEDIUM. A mill producing 100% recycled corrugating medium was using a conventional detackifier and topical treatments on the wire and paper machine rolls to control stickies and pitch. A cationic copolymer coagulant and starch were used along with a cationic acrylamide retention aid as strength and retention programs in the wet end. The furnish consisted of old corrugated containers (OCC) and mixed office waste (MOW). Due to stickies deposits, a maximum of 25% MOW could be used in the furnish. However, using more than 25% would lower fiber costs.
The initial objective was to maintain equivalent pitch and stickies control as measured by press and rewinder roll deposits, while also maintaining overall runnability. The structured protein was added, replacing the traditional detackifier. During the next week, deposits and runnability remained the same or were improved. However, the amount of topical treatment required was lowered by approximately 30%, and stickies deposition at the rewinder was reduced.
Measurement of the suspended solids and turbidity of filtrate from the machine chest and headbox indicated there was a "cleaning up" of the colloidal material in the system as well. The retention of suspended solids tracked closely with increased and decreased addition of the structured protein. A decrease in suspended solids in the presence of the structured protein indicates increased retention (Figure 1 and Figure 2). Correspondingly, an increase in filtrate suspended solids occurred with a decrease in structured protein addition.
|
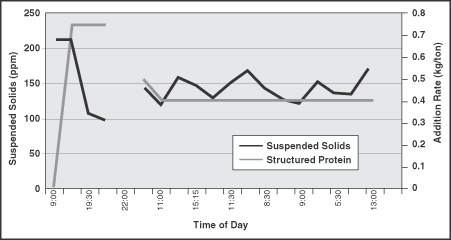
FIGURE 1. The level of machine chest colloidal contaminants closely tracks structured protein addition.
|
|
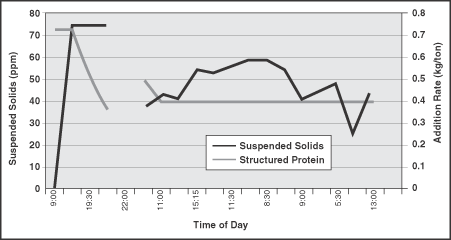
FIGURE 2. Colloidal contaminant levels in the headbox also tracks structured protein addition.
|
Following these positive initial results, and optimization of the feed rate, the MOW content was increased from 25% to 28%. Above 28%, sheet strength characteristics became a limitation, but pitch and stickies deposition was no longer the limiting factor.
CASE HISTORY: FINE PAPER. An integrated alkaline fine paper mill was experiencing a high frequency of wet and dry end breaks. The mill also had blue-tinted deposits in the stock chests and white water system. The deposit was determined to be a mixture of natural pitch, hydrolyzed alkyl succinic anhydride (ASA), and oil. The furnish consisted of hardwood and softwood bleached kraft pulp, precipitated calcium carbonate, ASA size, cationic starch, and a retention aid.
The structured protein was fed to the total furnish at a rate of 1.1 lb/ton. Total breaks per day decreased significantly with the addition of the structured protein (Figure 3). Additionally, there was no continued build up of the deposit on the machine.
|
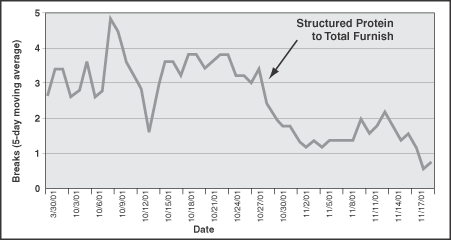
FIGURE 3. Paper machine breaks dropped following the addition of the structured protein to the paper machine.
|
CASE HISTORY: TISSUE. A 100% recycled tissue mill producing bleached and unbleached towel and napkin grades had a significant stickies problem. Production was being lost or downgraded, and solvent washes were frequently required. The mill used a PAE wet strength resin and a cationic copolymer for strength development and coagulation. In an attempt to control deposits, a number of continuous preventative treatment programs were employed. Despite these programs, this mill averaged 6.6 washes per day, and solvent use averaged 11.0 lb/ton.
The mill conducted a laboratory evaluation of the structured protein. A scanning electron microscope (SEM) was used to examine stickies that had been isolated from untreated and treated furnish samples. Stickies from the untreated furnish accumulated filler and fines, indicating a tacky, adhesive surface, while stickies from the treated furnish were clean and smooth, indicating a detackified surface.
Next, a trial was conducted using 1.2 lb/ton of structured protein. During the next ten days, solvent use was reduced by 64%, machine cleanliness improved, and salable production increased. To stabilize potentially smaller stickies particles, the addition point of the structured protein was relocated. Solvent use and the average number of solvent washes per day were reduced further with the new addition point (Table 1).
Additional benefits realized at the conclusion of the trial included reduced solvent discharge in the mill effluent, improved air quality around the machine, and cost savings related to increased production, reduced solvent usage, and improved runnability.
CHARLES D. ANGLE is applications group manager, contaminant control, for Hercules Incorporated, Pulp and Paper Division, Norcross, Ga.

|