Careful attention to detail when handling, cooking, and using starch in applications in the mill can minimize common problems such as starch spoilage, retrogradation, and contamination
|
 |
Good Housekeeping, Proper Preparation are Key to Papermakers’ Efficient Use of Starch
|
|
|
By BOB KEARNY
|
From the wet end to the size press to coating formulations and the calender stack, starch is used throughout the mill in large volumes. Starch enjoys such popularity because it provides a number of benefits and the potential to significantly reduce total costs. Wide availability and low, stable prices are a major reason behind the pervasive use of starch products.
Starch consumption in the North American paper industry has reached an annual level of 3.8 billion lb since it serves a variety of papermaking requirements. Most starch is now shipped in bulk, replacing the need for labor-intensive manual handling when shipped in bag form.
Mills deal with a variety of issues when shipping and handling starch, as well as when preparing and using it. Papermakers can encounter problems with cooking, spoilage, retrogradation, contamination, cleaning pipes and equipment, and handling. This article discusses procedures associated with these tasks and offers suggestions for minimizing problems.
IMPROPER STARCH COOKING. Improper starch cooking or hydrating is the biggest problem associated with starch use. This is true for all starch types and sources and for all applications in the mill.
Complete hydration of a starch molecule requires four things: water, temperature, time, and agitation. The amount of water needed depends on the type of starch and how it has been modified. For example, a wet end starch may require cooking at 6% solids, while a highly modified coating starch may cook at 40% solids.
 |
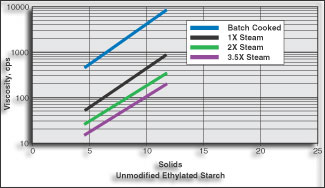
| FIGURE 1. This example of cooking unmodified ethylated starch clearly shows the effect on viscosity and solids of higher temperatures during high shear starch cooking. |
   |
|
Cooking solids are very critical to starch performance: If the solids level is too high, the performance of the starch will degrade. Shear is also important in order to completely explode and disperse the starch granules. In atmospheric cooking, it is necessary to maintain good high shear throughout the cooking process.
Most starch begins to gel between 140 and 160ºF. Highly modified starch begins to gel at temperatures as low as 115ºF. Some cross-linked starches require elevated jet cooker temperatures, for example, up to 195ºF or higher. Starch cooked at atmospheric pressure may require a 20- to 30-min cooking time, while cooking is instantaneous in jet or thermal/chemical cooking processes.
The most common methods of cooking are atmospheric or batch, enzyme, jet, and thermal/chemical.
Enzyme conversion. The enzyme conversion process consists of making up slurry of water and starch at the desired total solids and adjusting pH to the recommended value. The slurry is agitated and heated at a programmed temperature rate rise until about 170ºF. After holding there, usually for about 30 min, the temperature is increased as rapidly as possible at a programmed rate to about 195ºF. This temperature is usually adequate to “kill” the enzyme in about 15 to 30 min. The material is then cooled to the desired temperature.
In both batch and continuous enzyme cooking, strict control of several key factors cannot be overemphasized. These include the rate of rise in temperature, holding period, and viscosity. These factors require strict regulation in order to develop reproducible, uniform results.
The advantages of the enzyme modification or viscosity reduction process are its relative simplicity and low cost. However, as is the case for enzyme and other methods employed by the mill for viscosity reduction, problems can occur. For example, when modifying a product to obtain a low viscosity level as required by applications such as the size press, problems, such as a high soluble content, a reduction in the strength of the converted starch, and setback, usually occur.
Thermal conversion and jet cooking. Jet cooking is the preferred method for hydrating starch, and continuous cookers have been available for years. High-temperature, pressure, and high shear conditions are applied through the use of “excess” steam. This method provides considerably lower viscosity for a given starch compared to atmospheric cooking. Starch paste produced by jet cooking provides the following advantages: (1) a reduction in manpower, (2) automated cooking process, (3) uniform viscosity, and (4) complete hydration of the starch molecules.
The effect of steam on high shear cooking of unmodified ethylated starch is illustrated in Figure 1. The reduction in viscosity is due to extremely high mechanical energy imposed by the system in conjunction with complete hydration of the starch molecule. There is little to indicate that any breakdown of chain length occurs in the thermal process.
Slightly modified starches are used to provide viscosity/total solids relationships satisfactory for most coating applications
Chemical conversion. High-temperature chemical conversion is a continuous process involving an automatic chemical conversion system that mostly uses ammonium persulfate as a viscosity modifier. The starch slurry is pumped through a mixing jet where high-pressure steam elevates the temperature and creates shear. A holding coil follows to maintain the elevated temperature and pressure.
Adjusting the amount of chemical applied, along with the temperature, pH, and holding time of slurry in the coil, controls viscosity reduction through a wide viscosity range. However, while automated and relatively simple, the process has disadvantages.
For example, control of pH is very important. Discoloration of starch paste can also be a serious disadvantage and affect final brightness. Setback characteristics of the starch paste are also a disadvantage, and many different products have been promoted to reduce retrogradation problems and provide stability.
STARCH SPOILAGE. Starch and coating formulation deterioration or spoilage has been a problem in the paper industry for years and can cause production and finished product quality problems. Indicators that spoilage is occurring include loss of viscosity, lower pH, odor, and color change.
Research in recent years has shown that microorganisms are present in the aqueous phase. Excessive activity or growth of these undesirable bacteria and fungi are a direct cause of spoilage. However, starch as received from the manufacturer generally has a low level of microbiological contamination. While not sterile, bacteria counts in these products are low and will not multiply due to a lack of moisture. The osmotic pressure present in commercially dried starch is such that it prohibits growth. Thus, starch can be stored indefinitely if kept dry.
 |
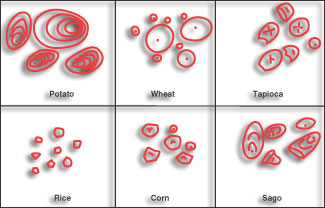
| FIGURE 2. The unique appearance of various starch types when examined under a microscope allows easy identification by the mill in order to avoid errors. |
   |
|
Mill water is the single biggest source of microbiological contamination. Fresh water used in mill operations sometimes has large masses of clearly visible growth. Spoilage-causing bacteria and fungi, however, are microscopic. River water may contribute abundant contamination from pollutants and organic byproducts. Lakes, ponds, creeks, and well water can also add contamination.
Chlorination or other fresh water treatments for bacteria are almost always advisable for reducing bacteriological content, as well as providing a bacteria-killing solution. This is useful during washups and for stock dilution and makeup. Typical bacteriological counts in river water vary from 200 to 100,000 colonies/ml. As an example, straight chlorination with a free chlorine residual of 0.5 ppm was used to reduce the average bacteriological count in raw river water at a newsprint mill from 68,000 to 197 colonies/ml.
Proper conditions alone can cause organisms to multiply and cause spoilage. Factors encouraging growth include moisture, food sources, temperature, time, hydrogen-ion concentration, and oxygen.
Moisture. The metabolism of microorganisms is similar to that of human beings in that moisture is essential. Spoilage can occur in starch (or paper) exposed to high relative humidity and wet storage conditions. A relative humidity of 85% or greater provides enough moisture to promote growth.
Food sources. Starch is an ideal food source for microorganisms. Once it is slurried, microorganisms begin attacking, and cooked starch is a perfect growth media.
Temperature. Each microorganism has an optimum temperature range for growth, which is normally 50 to 120ºF, but some grow and cause spoilage from 32 to 150ºF. Growth increases with temperature up to a maximum point.
Time. Growth is also directly affected by time. Microorganisms grow rapidly at optimum temperatures, and certain bacteria can double in number in as little as 16 min. Growth rates are much slower outside of the optimum temperature range.
Hydrogen-ion concentration. Bacteria normally grow at 4.0 to 9.0 pH, with highest activity at the middle levels of 6.0 to 8.0 pH. There is also enough growth at extreme pH levels to pose problems.
Oxygen. Most bacteria use oxygen in the air to grow (aerobic bacteria). In contrast, anaerobic bacteria cannot use oxygen in air and secure oxygen from food sources. Some, like facultative microorganisms, take oxygen from either source.
Aerobes appear on the starch surface or in coating tanks, but anaerobes exhibit subsurface growth such as found in tank bottoms, pipes, and dead-end equipment. This is important to consider when sampling for microbiological contamination.
COOKED STARCH SPOILAGE. Susceptibility to spoilage varies from starch to starch. For example, hydroxyethylated starches are resistant to attack because of ethylene oxide substitution. However, even they will support microbial growth during storage. Enzyme- and AP-converted starches are most susceptible to attack because there is no inhibiting chemical present. In enzyme-converted starch, the heat used to deactivate the enzyme system usually eliminates all of the enzyme. However, many times this is not the case and the enzymes continue attacking the starch.
In applications, such as clear size press and coating starch, a change in viscosity and pH normally indicates a microbial attack. Bacterial growth splits the starch molecule, causing lower viscosity, and the acid produced lowers the pH. The acid can act on clay in the coating and lead to an increase in coating viscosity. Spoilage is generally due to aerobic bacteria.
Good housekeeping and the use of preservatives are the most important means of spoilage control. It is imperative that mills have cleaning and sanitation programs for their starch systems to prevent spoilage. In addition, fresh batches of starch should be prepared once per eight-hour shift as a means to protect product quality.
The cleaning and sterilizing effect of hot water is one of the most effective methods of keeping equipment free of excessive microorganisms. For years, starch solutions have been held at 190ºF to prevent deterioration and formation of “papermakers amylose.”
During boil-outs, hot water and detergents or dispersants should circulate through the equipment, with particular attention paid to hard-to-get-at dead spots like elbows and valves. This cleaning makes it possible to start with an essentially contaminant-free system. Regular, periodic cleaning maintains this condition.
CHEMICAL CONTROL. Chemical control incorporates a preservative in either the starch slurry, cooked starch, or coating formulation. In all cases, dosage should be sufficient to control the spoilage organisms but not necessarily to eliminate them. The economics of chemical control dictate specific treatments.
Preservatives currently in use fall into the following classes: organo-sulfur compounds, organo-halogens, phenolic-type compounds and salts, heterocyclic nitrogen compounds, ester of organic salts, quaternary ammonium compounds, inorganic salts, miscellaneous organic compounds, and organo-mercury compounds.
The 1958 Food and Drug Administration (FDA) Amendment on food additives not only effects the choice of preservatives in food packaging, but in other areas. Non-food-grade mills may also use chemical preservatives and levels of addition that are suitable for food-grade production. Because of factors, such as FDA approval, changing effectiveness, and new products, the papermaker’s chemical supplier should be contacted for specific recommendations.
Chemical selection should be based on the microorganisms present, methods of mixing, temperatures involved, holding time requirements, susceptibility of coating ingredients to spoilage, and coating specifications. It is important to note that the application technology used for each preservative should guide the method, level, and point of chemical addition.
Preservative chemicals may exhibit undesirable effects that should be investigated prior to use. Examples include a change in color (possibly caused by high pH); odor; or an effect on emulsion, starch, or coating stability.
STARCH RETROGRADATION. Sludge formed in starch and storage tanks, which is the result of reassociation of the starch molecules, is sometimes called “amylose.” To differentiate this from amylose found in nature, it is referred to as “papermakers amylose.” This material, which is a product of starch retrogradation, is crystalline in nature, has a double helix structure, and exhibits a degree of polymerization (DP) range of 50 to 120, with the optimum DP at 100.
 |
| Starch is normally shipped in large bulk containers or bags, such as the one shown here feeding the mill’s slurry makedown tank. |
   |
|
When starch is suspended in water, agitated, and then heated over a period of time, it combines with water molecules (hydrates), increases in size, and immobilizes much of the water present. Thus, the viscosity of the mixture increases.
As the process continues, the granules become fragments, the individual molecules disperse, and the viscosity decreases. Also, viscosity increases as the temperature drops. In some instances, the straight chain molecules may line up parallel to each other and even precipitate from the solution. This recombining, realignment, and reassociation is called retrogradation. Because of its structure, the amylopectin portion of starch exhibits little retrogradation.
CONTAMINATION. A seemingly simple but important problem faced by mills is when different types of starch are mixed up, or in other words, contaminated. For example, a starch shipment may be inadvertently off-loaded into the wrong tank. Ensuring the right materials are in the right place is important. Table 1 provides a guide to using stains to identify starch.
In general, starch is identified by a few simple characteristics, including biological source, charge (if any), and cooked starch viscosity. Other characteristics like moisture and pH are used to further classify a particular starch. There are many other starch characteristics, so these factors are not sufficient to identify every single sample.
Common biological starch sources in the U.S. are yellow dent corn, waxy maize corn, potato, wheat, and tapioca. A simple check is to slurry uncooked starch in water and view it under a microscope. Such examination can be used to identify starch since each has a unique appearance, as Figure 2 shows.
Charged groups present on starch molecules are either cationic (positive) or anionic (negative). A starch may contain none, one or the other, or both charges. Charged dyes can be used to indicate the charge on uncooked starch granules but not on cooked starch.
The viscosity or, inversely, the fluidity of a starch sample can also be used to check for product contamination. Unmodified starches with no reduction in viscosity are often used in the wet end, but they can also be used in thermal-chemical and enzyme cooking processes for size press or coating applications. Extensively modified starches with very low viscosity are often used in coating applications where high solids are desired. Size press starch normally has a viscosity between these two extremes. However, because of the range of viscosities used in each application, viscosity is not always useful.
| TABLE 1: IN ORDER TO AVOID CONTAMINATION OR TO IDENTIFY AN UNKNOWN STARCH, STAINING IS ONE COMMON METHOD USED FOR STARCH TYPE IDENTIFICATION. |
| Starch Type |
Stain |
Identification Color |
Staining Procedure |
Regular Corn
(#2 Yellow Dent) |
Iodine |
Dark Blue |
Add two grams starch to 25 ml of 1% iodine solution.
Let stand five minutes and observe under microscope. |
| Waxy Maize |
Iodine |
Red |
Add two grams starch to 25 ml of 1% iodine solution.
Let stand 5 minutes and observe under microscope. |
| Cationic |
Fast Green* |
Green |
Add two grams starch to 25 ml of 1% Fast Green* solution.
Let stand 15 minutes; wash by filtration and resuspension until green color is absent from filtrate. Observe under microscope. |
| Oxidized |
Methylene Blue |
Blue |
Add two grams starch to 25 ml of 1% methylene blue solution. Let stand 15 minutes; wash by filtration and resuspension until blue color is absent from filtrate. Observe under microscope. |
| *Use either Kodak Light Screen SR, Yellowish (cert.) or Fisher Scientific Co. Fast Green FCF. |
|
PIPE LINE AND EQUIPMENT CLEANING. Paper mills can have trouble with pipes and other equipment that become caked with deposits, but cleaning solutions have been developed that help remove them.
Caustic cleaning solution. Starch deposits not containing polyvinyl alcohol can be removed by flushing with a hot 5 to 10% percent solution of caustic soda in water. It should be circulated through the lines at a temperature of 195ºF. This may involve special piping since the solution works more effectively if it is pumped continuously through the system rather than allowed to sit quietly. This hot solution must be handled safely.
Acid cleaning solution. Thick deposits build up in pipes and tanks if polyvinyl alcohol is used in the adhesive. Pumping a boiling hot, 5%-inhibited muriatic acid solution through the system for several hours can remove them. Removal of thick deposits may need up to 10 or 12 hours. If the acid cleaning solution is unavailable, mills can purchase the inhibitor and add it to regular muriatic acid.
STARCH SLURRY PUMPING. Slurry pumping equipment and operating conditions have an important effect on starch slurries. Excessive temperature causes the starch to begin the hydration process. This causes viscosity to increase (sometimes greatly), so slurry should be pumped below 100ºF. Very caustic conditions also cause the starch to hydrate.
Starch slurry is made up of native starch granules mixed with water below 100ºF. The opaque slurry is milk colored, and the granules will settle out. Granules range in size from 5 to 20 microns for cornstarch and 15 to 100 microns for potato starch. The pH will usually, but not always, range from 6.0 to 7.5.
The specific gravity of the slurry depends on the amount of dry substance starch mixed per gallon of water. Starch at 35% solids has a specific gravity of approximately 1.157 and has about 3.374 lb of dry starch/gal. The specific gravity of 38% slurry is about 1.172 (refer to starch Baume tables for further data). The viscosity of starch slurries below 35% is normally under 100 cps, while starch slurries over 35% solids can increase in viscosity. Slurry solids over 38% are not normally recommended.
Centrifugal pumps are usually most suitable, although positive displacement pumps can be used. With positive displacement pumps, it is important that the pump supplier acknowledges that the pump will handle low-viscosity fluids. All wetted parts should be 316 stainless steel. In order to prevent settling, a line velocity of 5 ft/sec or greater is recommended.
Pumps must be able to flush and drain since starch can block the impeller on startup if allowed to settle in the lower part of a pump volute. “Dead heading” a running centrifugal pump can cause the starch to hydrate from the heat of friction, turning it into a paste or gel. Pump seals can consist of packing, single, or double mechanical seals. Starch can also get into seals and plug seal water lines.
STARCH FILTRATION. Starch slurries and pastes should be filtered or screened. When used properly, filtration reduces downtime and process variability and increases productivity. The slurry should be strained prior to the starch cooker. Size press solutions, coatings, or pastes should be filtered just before the paper machine. Starch used on the size press or at the coating head often undergoes coarse screening before re-entering the run tank. Woven, perforated, or slotted screens are used, depending on the application. Filters should be inspected and cleaned on a regular schedule.
The following are suggested characteristics for starch filtration equipment: (1) 316 stainless steel for wetted parts, (2) filtered water supplies, (3) coarse basket strainers for protecting cookers and finer filters for protecting processes, (4) 20 to 40 mesh filters for slurry, (5) 60 to 150 mesh filters for paste, (6) slotted-type filters where fiber is present, and (7) pre-screening where oversize contaminants can enter a system (for example, at the size press).
BOB KEARNEY is manager of market development for Western Polymer Corp., Aenkeny, Iowa.
|