|
Chloride dioxide bleaching is increasing in popularity, but there is still plenty of room for improvement in the process
By Christine Chirat and Dominique Lachenal
Brushing up on bleaching techniques
Across Europe and North America, kraft pulp mills are opting to use chlorine dioxide as a substitute for chlorine. In the future, chlorine will be progressively phased out and at least partly replaced by chlorine dioxide in all major pulp producing countries. The trend toward using chlorine dioxide represents a significant investment cost for mills as a new chlorine dioxide generator needs to be installed on site. The process also leads to considerably higher operating costs as active chlorine is twice as expensive in chlorine dioxide stages as it is in chlorine stages.
Chlorine dioxide is used today in two types of ECF (elemental chlorine-free) sequence. Firstly, in conventional sequences such as D0 E D1 E D2 or O D0 E D1 D2, using 15 - 50 kg/(ODT) oven dried ton of pure chlorine dioxide (40 - 130 kg/ODT of active chlorine). And secondly, in 'ECF-light' type sequences such as O Q (PO) D Q (PO), or O O Q (PO) D0 E D1, using generally less than 10 kg/ODT of chlorine dioxide (or 26 kg/ODT of active chlorine).
Given the relatively high cost of chlorine dioxide and the large quantities which are required, there is a strong incentive to find ways of improving chlorine dioxide bleaching. Added to that, regulations on mill effluents may change and become more stringent in certain countries. The main routes that are open to mills are:
• increasing the delignification efficiency when chlorine dioxide is used at the beginning of the bleaching sequence (D0 stage)
•increasing the bleaching efficency when chlorine dioxide is used toward the end of the sequence (D1 or D2)
•further reduction in the mill's effluent load, particularly the AOX (adsorbable organic ;halogens) content.
Several research teams are currently working to improve these techniques all over the world. In France, the CTP (Centre Technique du Papier) and the EFPG (Ecole Française de Papeterie de Grenoble) believe that there is still room for improvement in chlorine dioxide bleaching and have come up with several ways to help mills get the best out of their bleaching.
Better beginning
According to the CTP and the EFPG, one of the main areas of improvement can be found in the efficient use of chlorine dioxide in delignification (in the D0 stage). With a typical D0 E delignification process applied on a 30 kappa softwood kraft pulp, the kappa number decreases from 30 to between 4-5. The chlorine dioxide charge required is 0.2 x kappa number = 6% active chlorine, or 2.3% for pure chlorine dioxide (ClO2). If the calculation is made on 100 g of pulp, the quantity of ClO2 is 2.3 g, which represents 0.034 mole. The redox reaction that takes place during delignification in a D stage shows that five electrons are exchanged:
ClO2 + 5e- + 4H+ Ë Cl- + 2H2O
This means that the DE process requires the exchange of 5 x 0.034 = 0.17 electron for a 25-26 kappa drop, ie 0.007 electron per kappa unit.
Going back to chlorine dioxide chemistry, the theoretical number of electrons exchanged to fully oxidize (and remove) lignin during DE (from kappa number 30 to 4-5) is 0.075 (ie 0.003 electron per kappa unit drop). This figure is about half of the total that is actually exchanged. The difference is very important and means that at least half of the chemical is wasted in other reactions. The same is true for other delignifying agents such as chlorine or ozone. One reason for the difference must be that the chemicals react with dissolved lignin moieties.
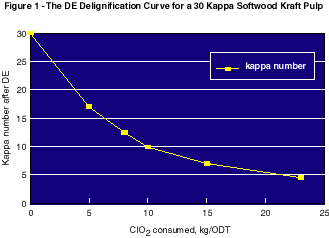
Figure 1 presents the DE delignification curve for a 30 kappa softwood kraft pulp. It can be seen that the kappa drop is not strictly proportional to the quantity of chlorine dioxide consumed. Delignification is more efficient at low chlorine dioxide charges and gradually slows down when the chlorine dioxide consumption increases. Taking the initial slope of this curve would give 0.005 electron exchanged per kappa unit loss, instead of 0.007 on the whole range of delignification. This value is still higher than the theoretical value of 0.003 though. The fact that the delignification slows down means that after a certain point, ClO2 starts to be significantly consumed by solubilized moities or side reactions.
Down with the AOX
Further improvements can be achieved by reducing the amount of AOX formed during chlorine dioxide bleaching. It is well known that the reaction of residual lignin in pulp with chlorine dioxide produces chlorite and hypochlorous acid simultaneously. When the pH is low enough, hypochlorous acid can be converted to chlorine. In most cases, a mixture of hypochlorous acid and chlorine is present during D stages and is responsible for chlorination reactions and consequently AOX formation.
A previous study2 showed that by adding dimethylsulfone (DMSO) during a chlorine dioxide stage, the AOX formed could be significantly reduced. The main reason was that the DMSO selectively reacted with chlorine and hypochlorous acid. The delignification efficiency of the DE stage was also affected as a result. This was probably caused by hypochlorous acid and/or chlorine directly participating in delignification during a D stage. The study showed that the AOX can potentially be reduced if appropriate ways can be found to capture some chlorine or hypochlorous acid.
End improvements
It is well known that the chlorine dioxide used in a last bleaching stage is not very reactive and must be applied in rather large quantities relative to the modest chemical work performed. Added to that, it has to be used in tougher conditions than in D0, typically at a temperature of 70-80°C for two to four hours. In this case, there is also room for improvement which could lead to reductions in the operating cost of a bleaching line. A better understanding of the chemistry involved in chromophore elimination is essential in this process though.

Potential improvements in chlorine dioxide bleaching come under the microscope at the CTP in France
Getting better
According to research carried out at the CTP and the EFPG, there are three main ways in which chlorine dioxide bleaching can be improved:
• (DoA) stages at high temperatures to improve delignification effciency
• combinations of chlorine dioxide with ozone (D0Z) or (ZD0) to decrease bleaching chemical costs and AOX levels
• splitting chlorine dioxide and controlling pH to reduce the formation of AOX.
In the first area, one way to improve chlorine dioxide delignification (in D0 stages) is to raise the temperature to 95°C and extend the retention time to two to three hours. These conditions far exceed conventional operating conditions which take place at 40-60°C with 30 to 60 minutes retention time. The rationale behind the traditional operating conditions is that chlorine dioxide's reaction rate with lignin is already very fast at room temperature and does not need to be increased. Some attempts have been made in the past to work at higher temperatures with the aim of reducing retention time though3. Previous studies have shown that a treatment with sulfuric acid at high temperatures could reduce the kappa number of a hardwood kraft pulp by several units4,5 and improve the following oxygen or hydrogen peroxide stages, provided that the time in the acid stage was sufficiently long4,6.
Working at high temperatures with long retention times in a D0 stage, the mill can take advantage of the low pH in D0 to perform a combined (DA) stage7. At high temperatures (95°C), the chlorine dioxide is totally consumed in less than a few minutes. In spite of this, the kappa number continues to decrease slowly during the retention time which is extended to one to three hours. The effect is much more pronounced with hardwood pulp than softwood. The results in Table 1 show that with hardwood kraft pulps, full ECF bleaching is easily performed while saving up to 20% chlorine dioxide. Hydrolysis of hexenuronic acids5 and, to a minor extent, acidolysis of residual lignin by the acidic medium are assumed to be responsible for this effect. Pulp quality is not affected provided that the pH of the D stage is kept above 2.
| Table 1 - Full bleaching of an oxygen delignified mixed hardwood kraft pulp (initial kappa n° 8.9) by D0 E D1. Effect of temperature in D0. |
| Temperature ºC |
45 ºC |
95 ºC |
95 ºC |
| CIO2 in Do, kg/ODT |
13 |
10 |
10 |
| Initial pH |
3.0 |
3.0 |
3.0 |
| Time, min |
90 |
15 |
90 |
| CIO2 consumed in Do, kg/ODT |
13 |
10 |
10 |
| Final pH |
2.5 |
2.3 |
2.3 |
| Brightness, D0E, % ISO |
75.9 |
72.1 |
73.3 |
| Kappa D0E |
2.1 |
3.4 |
2.2 |
| CIO2 in D1, kg/t |
7.0 |
7.0 |
7.0 |
| CIO2 consumed in D1, kg/t |
6.7 |
7.0 |
6.8 |
| Final brightness, % ISO |
89.3 |
87.1 |
89.2 |
| CIO2 consumed, total in kg/ODT |
19.7 |
17.0 |
16.8 |
| Viscosity, mPa.s |
20.0 |
20.0 |
19.0 |
Combined effort
The second measure that can be taken is to combine chlorine dioxide with ozone (D0Z) or (ZD0) to decrease the bleaching chemical costs. Both CTP and EFPG have carried out extensive research into the combination of chlorine dioxide (D) with ozone (Z) over the last decade8,9,10. In theory, 0.6 g of ozone is needed to exchange the same number of electrons as 1 g of chlorine dioxide1 in ZE and DE processes respectively. This is the displacement ratio which should be observed when all the chlorine dioxide is replaced by ozone. Figure 1 shows that in a D0 stage the delignification rate slows down with a chlorine dioxide charge of around 10 kg/ODT. As a result, when chlorine dioxide is partially replaced by ozone, the replacement ratio is expected to be even higher, since the ozone replaces chlorine dioxide when this chemical begins to be less efficient.
Trials at laboratory and pilot plants9,10 have shown that in most cases the replacement ratio is higher than 2 (2 g chlorine dioxide replaced by 1 g ozone). Mills are also catching onto the idea and have already started to use the process11. It is easy to calculate that in many cases, significant cost savings will be achieved by modifying a DEDED sequence into a (DZ)EDED sequence, or ODED into O(ZD)ED. Added to that, as less chlorine dioxide is used, the AOX level is also reduced.
Another application of this (DZ) or (ZD) concept is to use it at the end of the bleaching sequence where the last points of brightness are difficult to obtain and significant chlorine dioxide charges are necessary. Research has shown that ozone used in final bleaching stages can be more efficient9.
Split level
The last measure which can be taken to improve chlorine dioxide bleaching is to split the chlorine dioxide and control the pH level which leads to reduced AOX formation. Attempts to reduce the amount of AOX formed during D0 stage generally result in a parallel decrease in delignification efficiency, for example when additives are used2, 12.
One way to reduce the AOX, while maintaining the same delignification efficiency, is to apply chlorine dioxide in neutral conditions (pH 6-8) which generates chlorite. If the pH is allowed to decrease naturally, chlorine dioxide is regenerated from chlorite. The high concentration of chlorite favors a rapid reaction of chlorite with the hypochlorous acid which is efficiently captured and less prone to react with lignin2. Chlorine dioxide splitting coupled with this procedure is particularly interesting and the impact can be clearly seen in Table 2. The idea developed here is similar to that described for chlorination13. Lower AOX is formed by ClO2 splitting and adapted pH profile. At the same time, only a small loss in delignification efficiency is observed. It must be noticed that ClO2 splitting alone does not bring any modification in either delignification or AOX formation and that the decrease in AOX appears only when neutralization is performed at the beginning of each ClO2 phase (DnDnD).
| Table 2 - Reduction of AOX in CIO2 bleaching by CIO2 splitting and control of the pH profile |
| CIO2 charge, % |
2.4 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
| CIO2 consumed % |
2.4 |
|
|
|
|
|
|
| Initial pH |
7.0 |
7.0 |
3.2 |
3.1 |
7.0 |
7.1* |
6.8 * |
| Final pH |
3.0 |
3.6 |
3.2 |
3.1 |
3.5 |
3.5 |
3.5 |
| DE kappa number |
4.0 |
|
3.9 |
|
|
4.3 |
|
| Viscosity, mPa.s |
28.5 |
|
27.8 |
|
|
26.7 |
|
| AOX, kg/t |
1.21 |
|
1.26 |
|
|
0.70 |
|
* the pH was readjusted to 7 with NaOH before the addition of ClO2.
Softwood kraft pulp, kappa number 30, initial viscosity 29 mPas. ClO2 bleaching: total time 90 min, 3.5% consistency, 70°C. With ClO2 splitting, the phases lasted 15, 15 and 30 min respectively.
E stage: 3% NaOH, 5% consistency, 70°C, 60 min. AOX was measured after mixing D and E effluents. In the experiments with pH adjustment before D, NaOH in E was decreased so as to keep a 3% NaOH total charge. |
Final thoughts
When chlorine dioxide (and oxygen) are used exclusively in an ECF bleaching sequence, the oxidizing power of chlorine dioxide is not used efficiently. Up to 50% of chlorine dioxide can be wasted in useless reactions. As a result, there is room for significant improvement in the process. High temperature, long retention time chlorine dioxide stages, combination of chlorine dioxide and ozone and chlorine dioxide splitting with intermediate neutralization, are some of the ways in which the bleaching process can be rendered more efficient. In turn, these measures result in reduced AOX formation in some cases, or perhaps more importantly for some papermakers, they can also lead to cost savings.
Christine Chirat is the chemical pulping and bleaching group leader at the Centre Technique du Papier, France. Dominique Lachenal is the director of research laboratory at the Ecole Française de Papeterie de Grenoble, France
References
|
1. D. Lachenal and C. Chirat. About the efficiency of the most common bleaching agents. To be presented at the 1999 TAPPI Pulping Conference, Orlando, October 1999
2. M.J. Joncourt, P. Froment, D. Lachenal and C. Chirat. Reduction of the formation of AOX during chlorine dioxide bleaching. TAPPI Pulping Conference, Chicago, 1995.
3. S. Norden and P. Mellander. Advancing the chlorine dioxide process. 12th Sunds Defibrator International Technical Seminar, May 1996.
4. A.Maréchal. Acid extraction of alkaline wood pulps before or during bleaching : reason and opportunity. J. Wood Chemistry and Technology 13, 2, pp 261-281, 1993.
5. T. Vuorinen, J. Buchert, A. Teleman, M. Tenkanen, P Fagerstrom. Selective hydrolysis in ECF and TCF bleaching of kraft pulps. International Pulp Bleaching Conference, Washington DC, April 1996.
6. D. Lachenal, J. Papadopoulos. Improvement of hydrogen peroxide delignification. J. Cell Chem. Technol. 22: 5, pp 537-546, 1988.
7. D. Lachenal and C. Chirat. High temperature ClO2 bleaching of kraft pulps. International Pulp Bleaching Conference, June 1998.
8. D. Lachenal, M.T. Viardin, M. Muguet. Degradation of residual lignin with ozone. Application to bleaching. Nordic Pulp and Paper Research J., vol 6, pp 25-29, 1992.
9. C. Chirat and D. Lachenal. Other ways to use ozone in a bleaching sequence. TAPPI Journal, 80, 9, 209-214, 1997
10. C.Chirat, D.Lachenal, R.Angelier and M.T. Viardin. (DZ) and (ZD) bleaching fundamentals and application. Journal of Pulp and Paper Science, 23, 6, 289-292, 1997.
11. K.J. Finchem. Ozone, chlorine dioxide combination gains appeal in bleaching sequences. Pulp and Paper 72 (2), pp 53-57, 1998.
12. Y. Ni, G.J. Kubes, A.R.P. van Heiningen. Reduction of the formation of organically bounded chlorine during ClO2 bleaching. J. Pulp and Paper Science 20(4), p 103, 1994.
13. R.G.Hise. Split addition of chlorine/pH control for reducing formation of dioxins. Tappi Pulping Conference Proceedings 1989
|
|